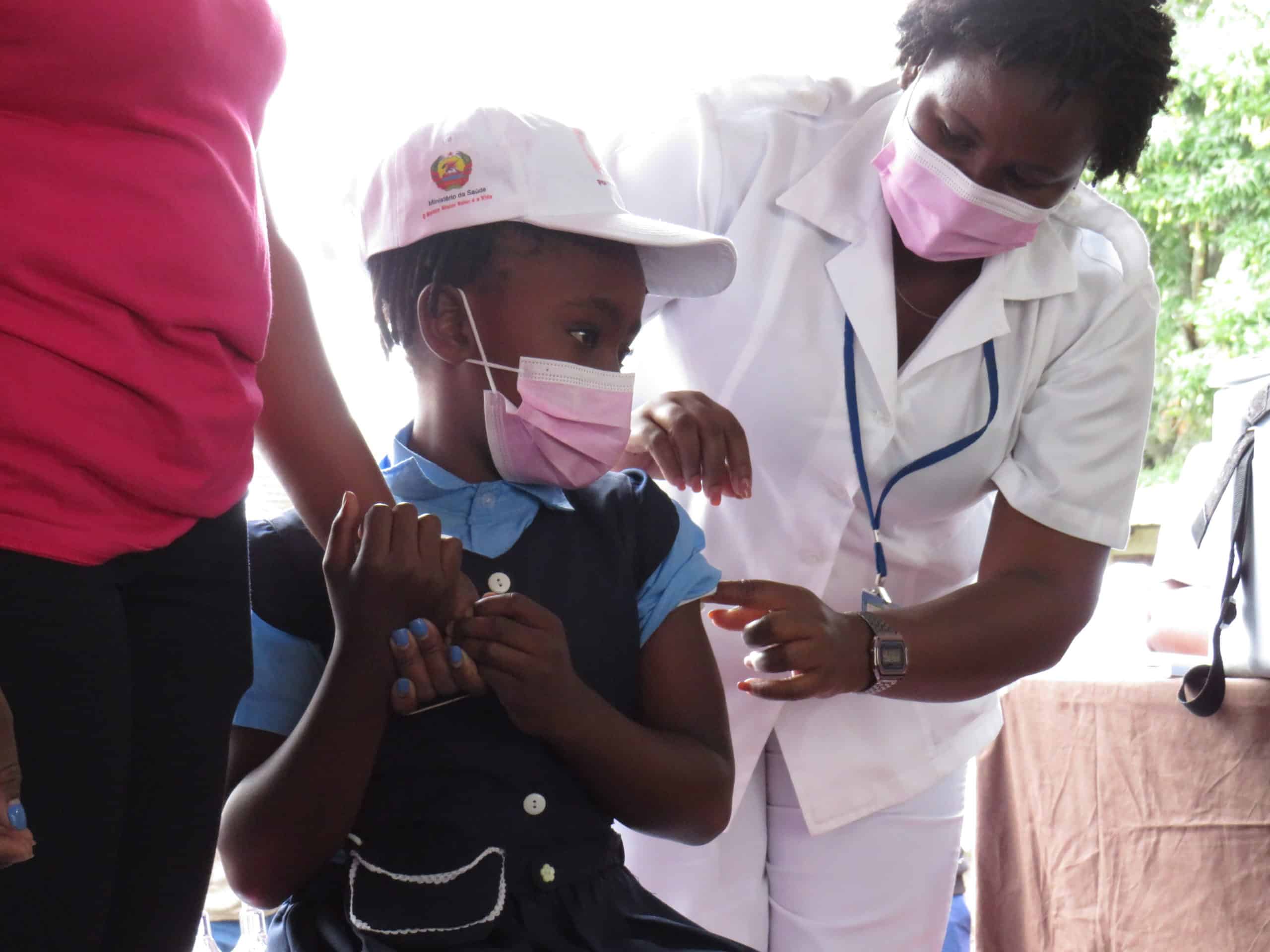
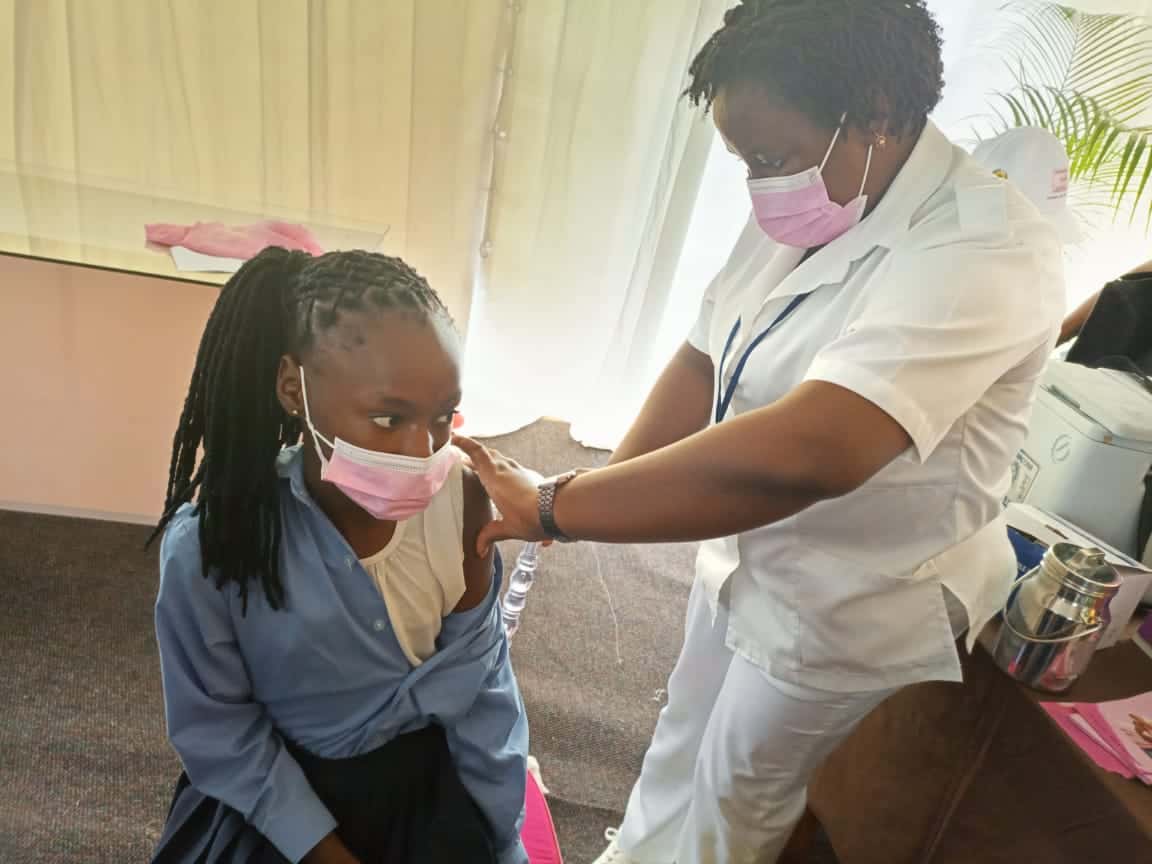
Globally, cervical cancer kills more than 250,000 women every year and 85 percent of these deaths occur in low- and middle-income countries. Two types of HPV cause 70 percent of cervical cancers and pre-cancerous cervical lesions. When provided to young girls between 9 and 14 years old, the HPV vaccine is the most cost-effective public health measure to prevent HPV and therefore cervical cancer.
Since 2013, with funding from Gavi, JSI has provided technical assistance to 8 countries introducing the human papillomavirus (HPV) vaccine nationwide. Vaccination efforts focused on reaching pre-adolescent and adolescent girls before they are at risk of exposure in Kenya, Cameroon, Zimbabwe, Tanzania, Madagascar, Malawi, and Mozambique. JSI also supported HPV vaccination demonstration projects in Niger, Madagascar, and Zimbabwe.
JSI provides technical assistance to countries with the planning, implementation, and monitoring of national human papillomavirus (HPV) vaccination programs. Our support includes preparation for the HPV vaccine grant application to Gavi, integration of HPV vaccination into country planning, multi-sector partner coordination and engagement (e.g. immunization, cancer prevention, education, adolescent health, civil society), and communication and social mobilization. JSI also provides technical assistance for training and capacity building of health care workers, analysis of HPV vaccine delivery and cold chain/logistics readiness and tracking, and supportive supervision and monitoring during and post-introduction.
JSI provides targeted technical assistance to HPV vaccine introduction based on the needs and national roll-out plans in each country, in close collaboration with the Ministry of Health’s Expanded Program on Immunization (EPI), Ministry of Education and/or other government programs, Gavi, WHO, UNICEF, and other partners.
JSI's in-country technical staff support activities such as preparation for the HPV vaccine grant application to Gavi; integration of the HPV vaccine into the country planning and routine immunization system; partner coordination and engagement; and communication and social mobilization. JSI also supports training and capacity building of health care workers, analysis of HPV vaccine delivery and cold chain/logistics readiness and tracking, and supportive supervision and monitoring during and post-introduction.
Through funding from Gavi, JSI currently supports the preparation and national introduction, as well as follow-up activities—including monitoring and national strategy evaluation—of the HPV vaccine in Cameroon, Kenya, Malawi, Tanzania, and Zimbabwe. JSI staff train and build the capacity of health care workers, teachers, and community leaders, analyze HPV vaccine delivery and cold chain/logistics readiness, and provide supportive supervision and monitoring during and post-introduction.